感谢您的支持,我会继续努力的!



打开支付宝扫一扫,即可进行扫码打赏哦
At present, the most common hydrogen sensors include catalytic type, electrochemical type, electrical type (metal oxide semiconductor, Schottky diode, etc.) and thermal conductivity type sensors, etc. In addition, because of the advantages of no spark, anti-electromagnetic interference ability, optical sensors are increasingly favored. Next, the author will introduce the basic working principle and main advantages and disadvantages of the above five types of hydrogen sensors one by one.
(1) Catalytic hydrogen sensor
Catalytic hydrogen sensors use the oxidation of gas on the surface of the electric heating catalytic element to detect combustible gas. This oxidation requires the use of oxygen in the air and releases heat causing the temperature of the sensing element to rise, depending on the concentration of the gas. The most common type of catalytic sensor is the "rheostatic type," which consists of two ceramic beads embedded with platinum wires. One of the ceramic beads is coated with a catalytic material that oxidizes when it encounters hydrogen, causing the temperature on the bead to rise, which changes the resistance of the platinum wire. At the same time, the platinum wire also acts as a heater, requiring the ceramic beads to be heated to a specified temperature beforehand. A Wheatstone bridge is needed to accurately measure resistance changes caused by temperature changes.

Element scheme of "rheostat type" catalytic sensor
Another common type of catalytic sensor is the thermoelectric sensor, which also takes advantage of the principle that hydrogen gas is oxidized to release heat, but uses the thermoelectric effect in the step of converting the heat signal to an electrical signal, rather than using the Wyeth bridge to measure the change in resistance caused by temperature rise. The catalytic hydrogen sensor has the advantages of mature technology, compact structure, small volume and very wide test range, but it also has obvious disadvantages. First, the catalytic hydrogen sensor is sensitive to any other combustible gas and cannot distinguish hydrogen from other combustible gas. Second, the oxidation reaction requires oxygen in the air, the explosion-proof performance of the sensor itself is poor; Third, catalysts can be poisoned by trace gases, such as silicone (i.e., polysiloxane) and hydrogen sulfide, and require regular calibration and replacement.
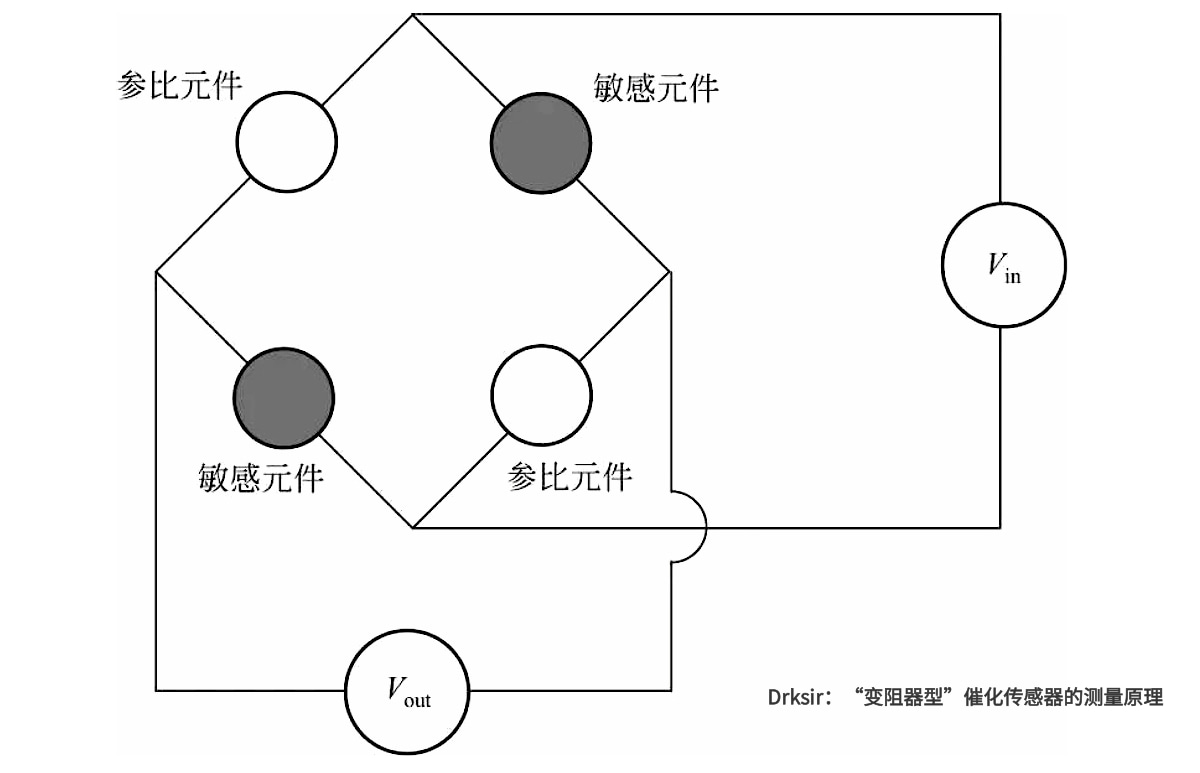
Measurement principle of "rheostat type" catalytic sensor
(2) electrochemical hydrogen sensor
Electrochemical hydrogen sensors can be divided into two categories: current type and potential type. The current type sensor detects hydrogen concentration by measuring the current generated by an electrochemical reaction, which occurs on the surface of the sensor electrode coated with a catalyst (such as platinum). In general, electrochemical hydrogen sensors have the metal anode and cathode immersed in an electrolyte (e.g., H2 SO4) to allow ions to transfer charge between the two electrodes. Because the current is proportional to the hydrogen concentration, the hydrogen concentration can be determined by measuring the current. Advanced electrochemical hydrogen sensors use a solid polymer electrolyte, which eliminates the risk of leakage when using liquid electrolytes.
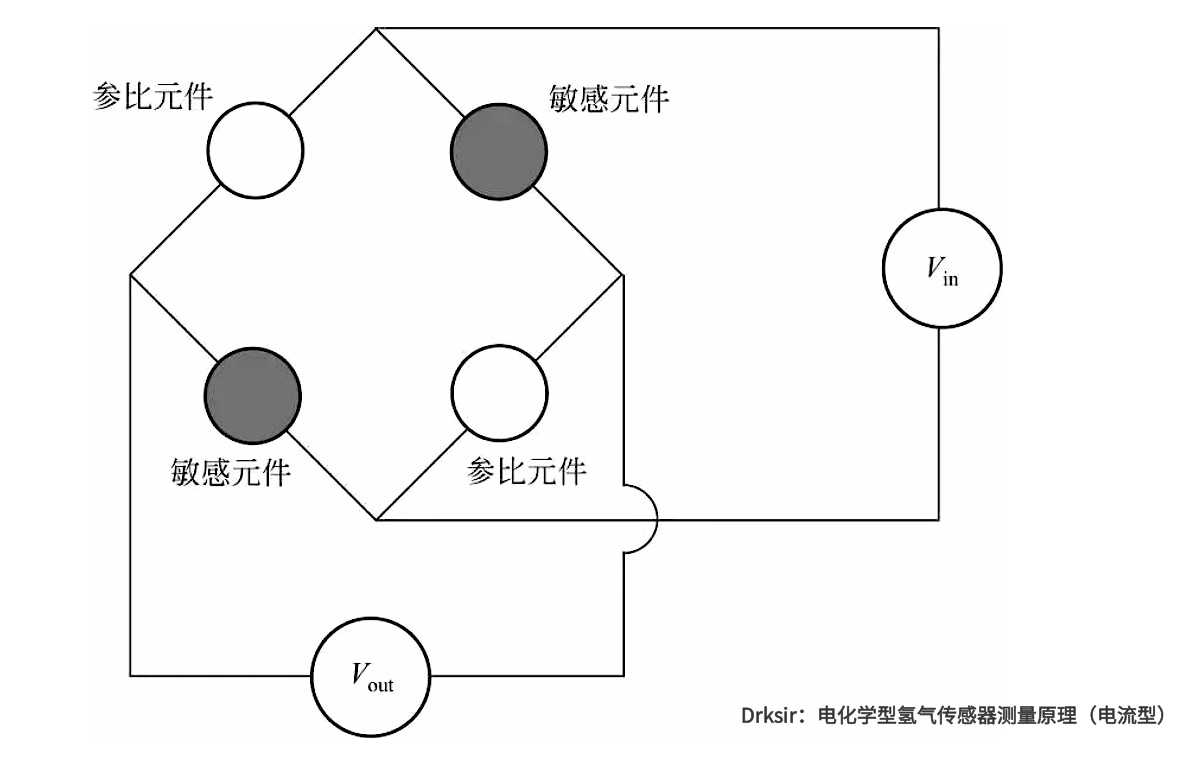
Measurement Principle of electrochemical Hydrogen Sensor (current type)
The difference between potential type sensor and current type sensor is that: current type sensor is working under constant voltage, sensor signal is current; The potential sensor works under zero current (open circuit), and the sensor signal is the potential difference between the test electrode and the reference electrode. Electrochemical hydrogen sensor has high sensitivity and accuracy, compact structure, and very small power consumption in the operation process, so it has preliminarily met the conditions for commercialization. At present, the main problem to be solved is the longevity problem -- electrode catalysts are easily poisoned by other gases in engineering applications, resulting in the accuracy of electrochemical hydrogen sensors will decrease over time. In addition, the narrow operating temperature is also the disadvantage of some electrochemical hydrogen sensors.
(3) Electrical hydrogen sensor
Electrical hydrogen sensors can be divided into resistive and non-resistive types. The former is typically represented by metal oxide semiconductor sensors, while the latter mainly uses Schottky diodes or MosFETs for measurement. Among them, the metal oxide semiconductor sensor is more common. The sensor has two electrodes. The substrate material between the electrodes is coated with a metal oxide film (such as tin oxide). The film acts as a hydrogen-sensitive material and its conductivity changes when it interacts with hydrogen gas (Figure 9-4). Thus, the change in semiconductor conductivity can be used as a measure of hydrogen concentration. The electric hydrogen sensor has the advantages of low cost, long life, low power consumption and miniaturization, so it has the potential of large-scale application. However, it is not selective to hydrogen, easy to be interfered by common gases such as water vapor, and has problems such as high operating temperature, slow start-up, non-linear, easy to be polluted.
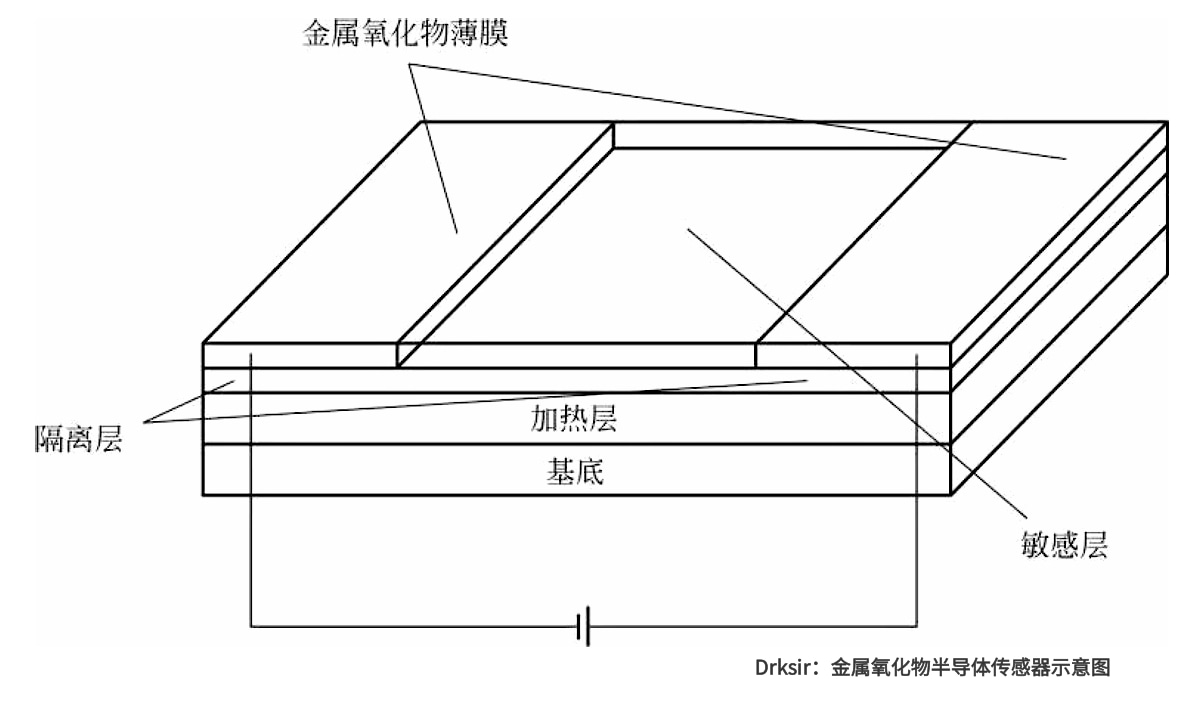
Diagram of a metal oxide semiconductor sensor
(4) thermal conductivity hydrogen sensor
The thermal conductivity hydrogen sensor relies on the high thermal conductivity of hydrogen for detection. Thermal conductivity is a unique property of each gas. Among all known gases, hydrogen has the highest thermal conductivity under normal conditions (around 273K and 101325Pa). Therefore, using air as a reference gas, hydrogen concentration can be determined according to the change of thermal conductivity. A schematic diagram of a heat-conductive hydrogen sensor is shown below. By measuring the thermal conductivity of the gas under test and comparing it with the reference gas, the concentration of hydrogen in the binary mixture can be determined. Two identical thermistors are used to convert the thermal conductivity signal into an electrical signal. One resistor is in contact with the gas to be measured and the other with the reference gas. The temperature (resistance) of a thermistor depends on the thermal conductivity of the surrounding gas, which is proportional to the concentration of hydrogen in the gas mix.
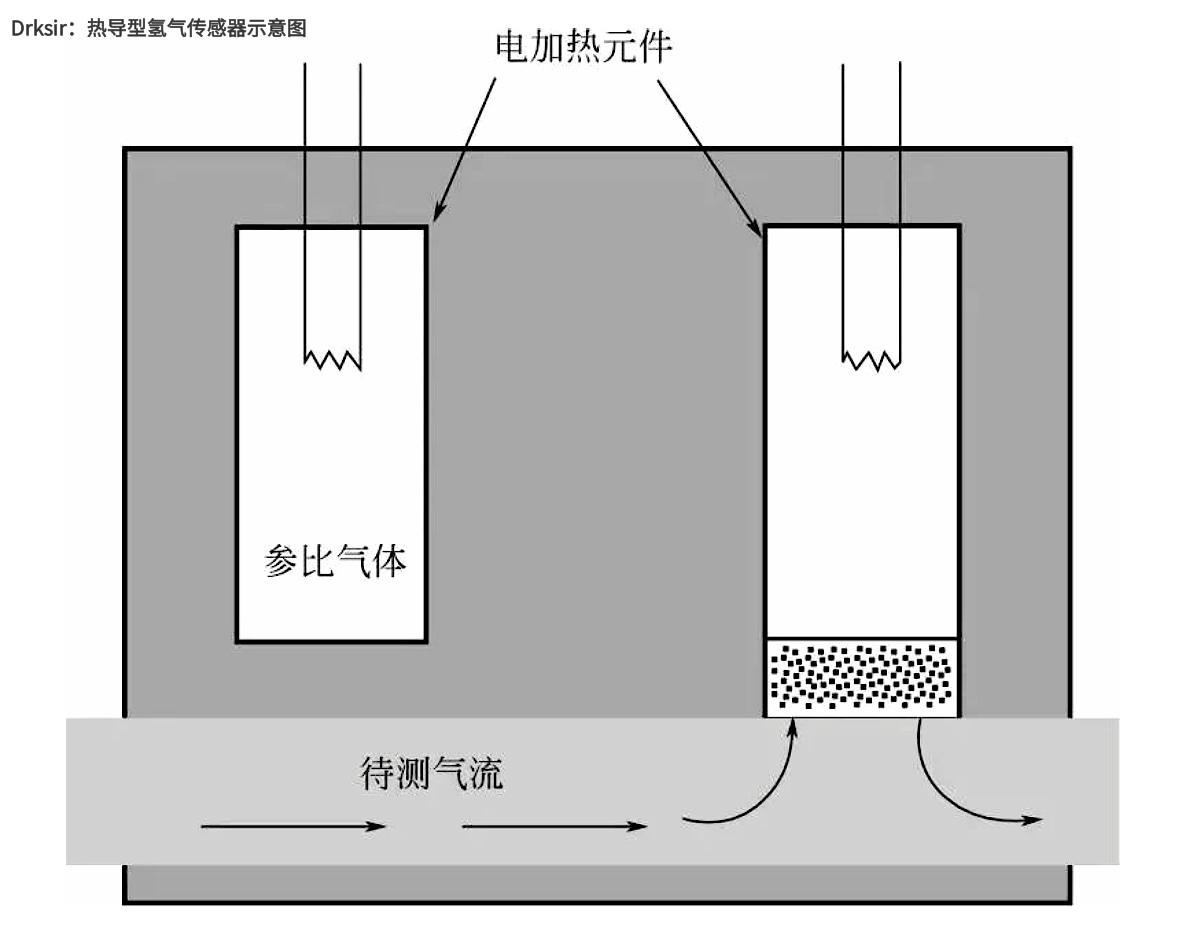
Schematic diagram of a thermal conductivity hydrogen sensor
Because there are no chemical reactions, the thermoconductive hydrogen sensor is relatively stable and has a long service life, and is particularly suitable for detecting high concentrations of hydrogen. However, at the same time, it is difficult to detect very low hydrogen concentration. It is usually necessary to use with other types of hydrogen sensors, or through sensor miniaturization technology to improve the above shortcomings.
(5) Optical hydrogen sensor
There are many types of optical hydrogen sensor, among which optical fiber hydrogen sensor is the most common. Fiber optic hydrogen sensor can be divided into microlens type, interference type, disappearing field type, fiber Bragg grating type and other types, but the basic principle is to combine fiber with hydrogen sensitive material, hydrogen sensitive material and hydrogen contact interaction, cause the change of physical properties of the fiber, and then change the optical characteristics of the transmitted light in the fiber. Finally, the hydrogen concentration is determined by detecting the change of a characteristic physical quantity of the output light. The most commonly used hydrogen sensor in fiber optic hydrogen sensor is palladium film. Different types of sensors take advantage of different physical quantity changes. For example, interferometric fiber hydrogen sensor takes advantage of the principle of volume expansion after palladium film interacts with hydrogen gas, stretching fiber, increasing optical path and then changing phase. The fiber Bragg grating hydrogen sensor also uses the principle of volume expansion after the interaction between palladium film and hydrogen, but it determines the hydrogen concentration by measuring the change of grating distance. The microlens fiber hydrogen sensor takes advantage of the principle that the reflectivity and refractive index of palladium change after the adsorption of hydrogen into palladium hydride.
Optical hydrogen sensor transmission signal is optical signal, there is no risk of becoming ignition source, so it is particularly suitable for use in flammable and explosive environment. At the same time, it also has the advantages of wide monitoring area, operating in anaerobic environment, anti-electromagnetic interference and so on. However, optical sensors are also susceptible to ambient light interference and are too sensitive to temperature changes.
summary
Hydrogen sensor is one of the key components in the field of hydrogen energy, which can quantify and detect hydrogen leakage. It is the core and foundation of hydrogen alarm device, and has great significance for improving hydrogen safety. At present, Drksir researchers have successfully developed a variety of hydrogen sensors based on different working principles, and many more promising and attractive hydrogen sensors are in the laboratory stage. However, at present, almost all types of hydrogen sensors have high cost, short life, anti-interference is not strong enough problems, there is still a certain gap from mass production, "into the thousands of households" requirements, such as Dexil's research and development strength of the team is not much. On the one hand, it is necessary to continue to optimize the existing sensor types. On the other hand, innovation should also be persisted to find new scientific principles that can be used for hydrogen sensor, so as to realize the breakthrough of hydrogen sensor as soon as possible.

关注公众号
了解更多传感器知识
公众号:德克西尔

加微信
购买传感器产品
微信号:Drksir-13515810281
